Protecting companies in a wide range of sectors
Explore allEnsure your cleanrooms reach the highest standard of sanitised production.


From preventing the spread of disease to ensuring the safety of your equipment, our healthcare cleaning solutions play a vital role in protecting your cleanroom environments.

Whether you’re manufacturing personal care products or lifesaving pharmaceutical products, we understand your need for safety, compliance, and efficiency within your cleanrooms.

Throughout all aspects of the public sector, we understand that your priority is keeping your people safe, which is why our range of commercial hygiene supplies make it easy to protect public spaces while remaining compliant.

Maintaining high cleanliness standards is key for your hospitality business, which is why our solutions ensure your customers remain safe and satisfied while you continue to meet regulatory standards.

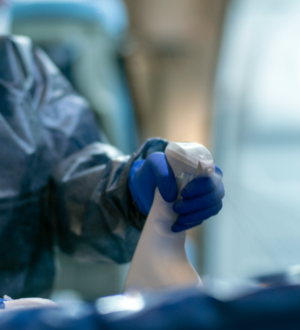
AGMA’s range of sterile and 0.2 micron filtered solutions ensure the highest standard of sanitation across all cleanroom environments.
With our products you can meet all cleaning and disinfection needs with just four different product types, we remove the need for unnecessary chemicals and complex procedures, helping you save on costs while optimising efficiency and ensuring compliance.
Neutral Detergent – Effective cleaning before disinfection
Disinfectant Alcohol – IPA and Denatured Ethanol
Sporicide – Zyceine
High Purity Water – WFI Quality Water for rinsing
Verified by independent efficacy data, all our products meet strict regulatory standards, enabling you to clean with confidence.
Where necessary, all AGMA products are blended with WFI quality water to help ensure low endotoxin levels. Unlike other disinfectant suppliers, we use our own WFI quality water to manufacture and blend our products to the highest standard, guaranteeing a specification of less than 10 cfu per 100ml water.

Each environment is different and requires various levels of cleanliness for operation depending on your processes. With regards to the requirements of GMP associated with medicinal production areas, the EU Commission states that “In Grade A and Grade B cleanrooms, disinfectants and detergents should be sterile prior to use.”
If servicing a Grade C (ISO Class 7/8) or Grade D (ISO Class 8) cleanroom, or an unclassified area, we advise you use the solution that works best for your environment. We provide a choice of both sterile and 0.2 micron filtered products developed for use in areas ranging from Grade A (ISO Class 4) cleanrooms to unclassified areas where 0.2 micron filtered disinfectants and detergents are required for a good standard of cleanliness and hygiene.
Use biocides safely. Always read the label and product information.

AGMA Ltd is a long established Chemical Formulator specialising in the manufacture of high quality, sterile and 0.2 micron filtered, cleaning & disinfection products for use within the Healthcare sector where the need to control pathogenic organisms is paramount. The development, formulation & provision of Biocidal products for this market is therefore at the core of our product offering and as such we manufacture a number of products affected by the Biocidal Products Regulations (BPR). BPR requires that all new and existing Biocidal products must obtain prior authorisation before they can be made available on the EU market meaning that AGMA Ltd. has to now obtain authorisations under BPR for its core product lines even though these products have been manufactured and sold worldwide for many years. In accordance with BPR all formulators including AGMA Ltd must purchase their Biocidal Active Substances from companies whose products are Article 95 listed under the BPR process. We are in close and constant dialogue with our Active Substance suppliers monitoring their progress through the Biocidal Products Registration process and AGMA Ltd. are in the process of preparing dossiers for our own product authorisations where appropriate. AGMA has consequently prepared it’s Dossier for it’s IPA product family group which was granted authorisation in 2021. Subsequently, a series of authorisations in a number of EU countries and the UK have been obtained. Please contact us for further information on the relevant EU countries.
The complexity and scale of costs associated with the BPR registration process across EU may lead to many formulated products becoming non viable commercially. However at AGMA Ltd. we are fully confident that our core product range can and will be maintained as our Directors took the decision to protect their core products supply chain by becoming part of the ASD Consortium for Alcohols and Article 95 for IPA, Denatured Ethanol and n-Propanol.
We are now undertaking a significant investment plan to ensure our core products a safe passage through the BPR registration process for the specific applications for which they are utilised by our key customers.
Social Care
Cleaning with care
Ensuring high standards of sanitation is a key component in securing the comfort and safety of your care staff, social workers, and those you care for. Our bespoke solutions keep your people protected while ensuring compliance.